BACKGROUND
Elranatamab(ELRA) is a BCMAxCD3 bispecific antibody being investigated for the treatment of relapsed/refractory multiple myeloma (MM). The phase 2 MagnetisMM-3 (MM-3; NCT04649359) trial was single-armed; the aim of this study was to contextualize the efficacy data from MM-3 with two real-world (RW) external control arms.
METHODS
We conducteda retrospective cohort study to indirectly compare the efficacy observed in MM-3 Cohort A (BCMA-naïve; N=123) from the March 2023 data cut (approximately 15 months of follow-up) with two US-based oncology electronic health record databases, COTA and Flatiron Health (FH), as external controls. MM-3 inclusion (eg, prior MM diagnosis, ECOG≤2, refractory to ≥1 PI, ≥1 IMiD, and ≥1 anti-CD38) and exclusion (I/E) criteria (eg, plasma cell leukemia, smoldering MM) were applied to each RW database to obtain comparable patient populations across sources. After imposing MM-3 I/E criteria, we conducted comparisons between data sources on progression-free survival (PFS) and overall survival (OS) using Kaplan-Meier estimators or Cox proportional hazard models (“unweighted analyses”) and restricted mean survival time (RMST) analyses in instances where the proportional hazards assumption was violated. We used additional Cox proportional hazard models, Kaplan-Meier estimators, or semi-parametric models implementing inverse probability of treatment weighting (IPTW), doubly robust, and propensity score (PS) matching analyses to adjust for any remaining imbalances across cohorts on confounding variables (eg, age, comorbidities, Eastern Cooperative Oncology Group (ECOG) score, International Staging System (ISS), prior lines/refractoriness, cytogenetic risk, extramedullary disease [COTA only], lab values).
RESULTS
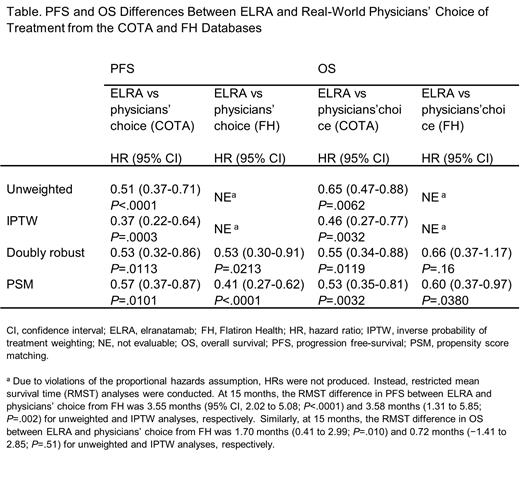
N=123 patients from MM-3 Cohort A were compared with the 239 and 152 patients identified from the COTA and FH databases, respectively. Treatment regimens in the RWD sources included various combinations of PIs (carfilzomib- and bortezomib-based regimens were each used by 43% and 15% of patients, respectively), IMiDs (lenalidomide- and pomalidomide-based regimens were used by 9% and 41% of patients, respectively), and mAbs (daratumumab- and isatuximab-based regimens were used by 32% and 1% of patients, respectively), among other agents (eg, selinexor-based regimens were used by 5% of patients). Across unweighted, IPTW, doubly robust, and PS matching analyses, ELRA was associated with significantly longer PFS than RW physicians' choice of treatment from both COTA and FH (hazard ratios [HRs] ranged from 0.37 to 0.57; see Table 1). Similarly, across unweighted, IPTW, doubly robust, and PS matching analyses, ELRA was associated with significantly longer OS than RW physicians' choice from COTA (HRs ranging from 0.46 to 0.65) and longer OS across unweighted (RMST difference at 15 months = 1.70) and PS matching analyses (HR = 0.60) than RW physicians' choice from FH. IPTW and doubly robust methods indicated only directionally longer OS for ELRA than RW physician's choice from FH (RMST difference at 15 months = 0.72 and HR = 0.66, respectively).
CONCLUSIONS
Among BCMA-naïve patients who resemble those enrolled in the MM-3 trial, those treated with ELRA exhibit significantly longer PFS and OS compared with real-world treatments.
Disclosures
Costa:BMS: Consultancy, Honoraria, Research Funding; Adaptive biotechnologies: Consultancy, Honoraria; AbbVie: Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Genentech: Research Funding. LeBlanc:Dosentrx: Current equity holder in private company; UpToDate: Patents & Royalties; Deverra Therapeutics: Research Funding; Agilix: Consultancy, Honoraria; Agios: Consultancy, Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Speakers Bureau; BlueNote: Consultancy, Honoraria; Incyte: Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; Duke University: Research Funding; American Cancer Society: Research Funding; Leukemia and Lymphoma Society: Research Funding; Novartis: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; CareVive: Consultancy, Honoraria; Meter Health: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; GSK: Consultancy, Honoraria, Research Funding; Flatiron: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; National Institute of Nursing Research/National Institutes of Health: Research Funding; Seattle Genetics: Research Funding; Servier: Consultancy, Honoraria. Sonneveld:Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Kyle:Pfizer: Consultancy. Hlavacek:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Schepart:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Aydin:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Nador:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. DiBonaventura:Pfizer: Current Employment, Current equity holder in publicly-traded company.